Tuesday 31st October 2023 Day 1
Wednesday 1st November 2023 Day 2
The Department of Biomedical Engineering (BME) organized a two-day workshop on "Recent Trends in Medical Device Design and Development," as the Pakistan Engineering Council (PEC) Continued Professional Development (CPD) event.
Day 2
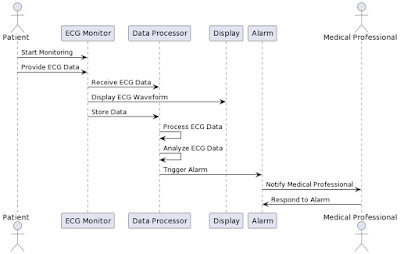
In computing lab 5, the event started with the recitation from the Holy Quran by Engr. Shahid Hussain, Sr. Lecturer BME. Engr. Tayyaba Khalid was the first speaker. The title of her presentation was, "Implementation Architecture for Medical Device Design." She explained the implementation model through a selected process. The role of the Biomedical Engineer in the Design Process, Verification through the use of Computer Simulation, and Validation through User needs were emphasized.
Engr. Dr. Muhammad Faris, Assistant Professor & Program In-Charge BME was the fourth speaker. The title of his speech was, "Quality Standards for Medical Device Manufacturing." He explained the selected standards used by the medical device manufacturers. The requirements specified by US Food and Drug Administration (FDA), Pakistan Standards and Quality Control Authority (PSQCA), and Drug Regulatory Authority of Pakistan (DRAP) were briefly outlined. Standards: ISO 14971, ISO 10993, IEC 62304, ISO 9001, ISO 13485, ISO 14001, ISO 50001, OHSAS 18001, and ISO 45001.








Very good summary Sir, Thank you for keeping us updated
ReplyDelete